Research

2 NOV. 2020, The embryos and us.
How multi-dimensional molecular architecture regulate cell fate decisions during mammalian embryogenesis? The Zhou Lab's long-term goal is to understand how cells regulate the commitment to specific lineage and how failure to execute terminal differentiation can underlie diseases. This understanding of cell fate and embryogenesis is a critical and overlooked facet of the teaching and practice of medicine and the conduct of biomedical research.
Conceptually, the Zhou Lab integrates omics profiling, genetic/epigenetic manipulation, and in vitro/vivo functional identification to link the genome with cumulative cellular phenotypes during embryogenesis. In particular, we are interested in understanding how changes in transcriptome and epigenome coordinate to shape the gene-expression landscape to form functional state of a developmental cell.
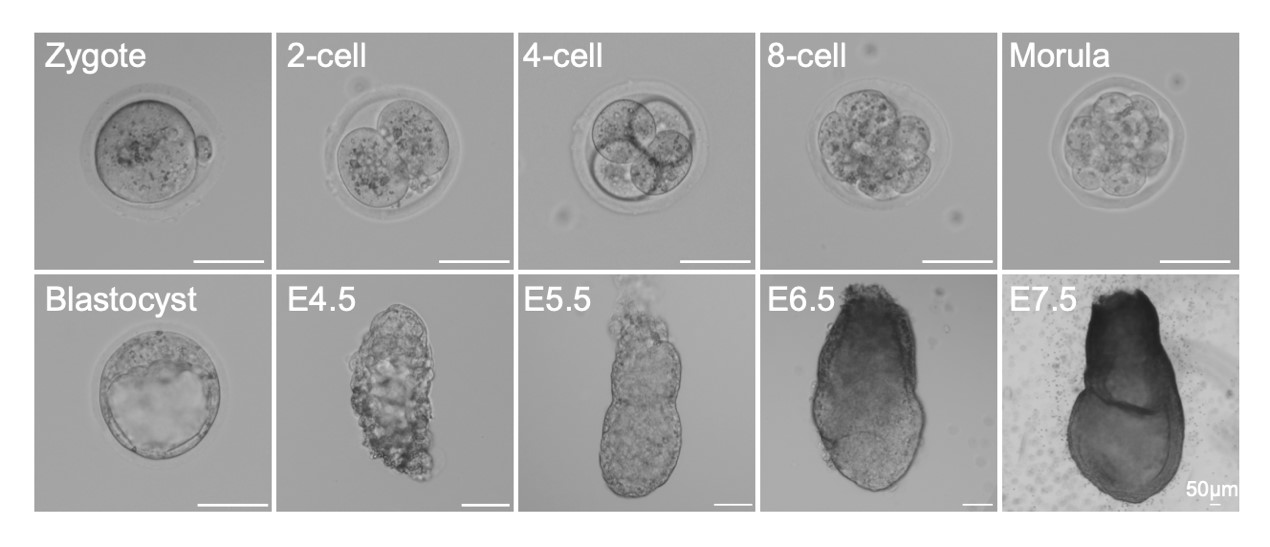
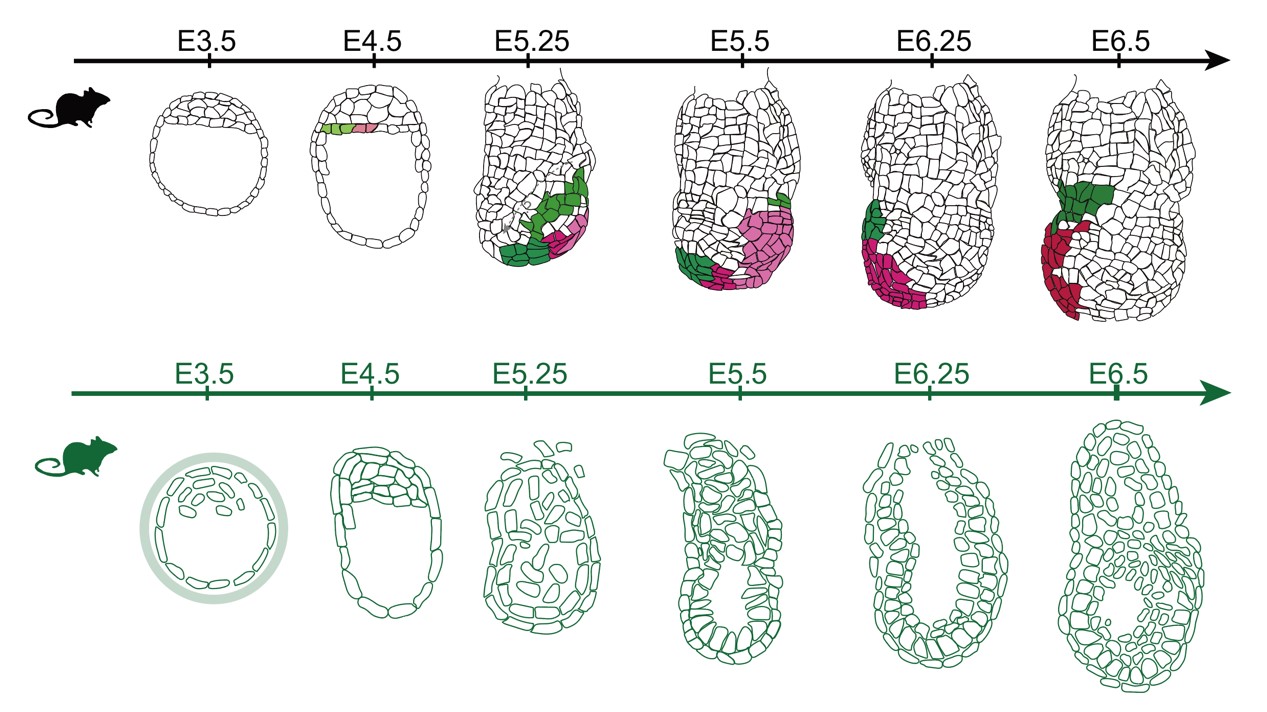
Physiologically and developmentally, connecting preceding blastocyst formation and following gastrulation respectively, peri-implantation embryogenesis is a key biological event during mammalian development. The embryo undergoes a series of cellular and molecular regulatory processes from pre- to post-implantation transition (PPT). The Zhou Lab is also interested in molecular transition, lineage specification, embryo patterning, the mother-offspring interaction and implantation activities. Our studies will positively enhance researcher's direct exploration of embryonic development in human beings and benefit reproductive medicine.
- Employing/developing single-cell multi-omics analysis to uncover the molecular patterns of lineage specialization during embryonic development;
- Establishing in vitro/vivo models to understand the regulatory mechanism of cell fate transition during embryogenesis from the phenotypic/functional dimension;
- Exploring the regulatory principles in embryo implantation and tumor evolution in reproductive system.